
Aid for Ukraine and Israel, possible TikTok ban pass Senate
The Senate passed the foreign aid package, which includes a provision that could lead to a ban on TikTok, after months of disagreement in Congress.
Watch CBS News

The Senate passed the foreign aid package, which includes a provision that could lead to a ban on TikTok, after months of disagreement in Congress.

Jurors in Donald Trump's trial in New York heard testimony from a former media executive about his efforts to bury negative stories about Trump before the 2016 presidential election.

It comes over a year after a shooting at a Nashville school killed three children and three adults.

The former officer, Elias Huizar, is suspected of killing two women and abducting a child in central Washington.

Former New York Rep. George Santos is no longer running for Congress, he announced Tuesday on X.

The Federal Aviation Administration said the aircraft was carrying two people at the time.

Larry Webb confessed to shooting and killing Susan and Natasha "Alex" Carter, who had been missing for 24 years, officials said.

Former President Donald Trump could receive a large windfall from his newly public media company, Trump Media & Technology Group.

Surprise guests, a broken foot and a history-making headliner.

Regulators prohibit new noncompetes, which impede millions of U.S. workers from getting a better job.

Ryan Watson could face at least 12 years in prison in Turks and Caicos after airport security allegedly found four rounds of hunting ammo in his carry-on.

At least 77 students from the women-only college at Cambridge University were recruited to the WWII code breaking station.

Trump's bid for sweeping immunity lands before the Supreme Court, which will hear a case Thursday over whether he can face federal charges related to an alleged effort to overturn the 2020 election.

This will be the first General Conference since more than 7,600 mostly conservative congregations left the United Methodist Church between 2019 and 2023.

The Netzah Yehuda Battalion of the Israel Defense Forces has faced criticism for its conduct. Will the U.S. take action?

Don Steven McDougal, a family friend, was indicted by a Polk County grand jury in connection with the death of an 11-year-old girl.

"He's ultimately responsible," former House Speaker Nancy Pelosi said of Israeli Prime Minister Benjamin Netanyahu.

Six men have been arrested on suspicion of involvement in the drug's transport, a Swedish customs official said.

Trump made 10 social media posts that were "threatening, inflammatory," prosecutors said, arguing he should pay a fine for each post.

Jurors in former President Donald Trump's criminal trial in New York got their first glimpse of the arguments both sides plan to make.

The case against former President Donald Trump stems from a "hush money" payment of $130,000 to adult film star Stormy Daniels in 2016.

Scammers have been increasingly successful in leveraging their romantic grip on victims by turning them into unwitting co-conspirators, or "money mules."

Laura Kowal's match on an online dating site wasn't what he seemed. Now her daughter is on a mission to expose the risk of romance scams: "It could happen to anybody."

Officials say the story of a woman found dead, her savings drained, after meeting a con artist on an online dating site is part of a national crisis unfolding largely in secret.

The RNC announced an ambitious initiative to monitor vote processing in the 2024 presidential election.

This appears to mean only a pro-abortion rights measure may qualify for the Colorado ballot this fall.

They backed the president even as their brother makes his own bid for Biden's job.

Protesters have been arrested at Columbia and Yale as they've refused to move, calling for a break from Israel.
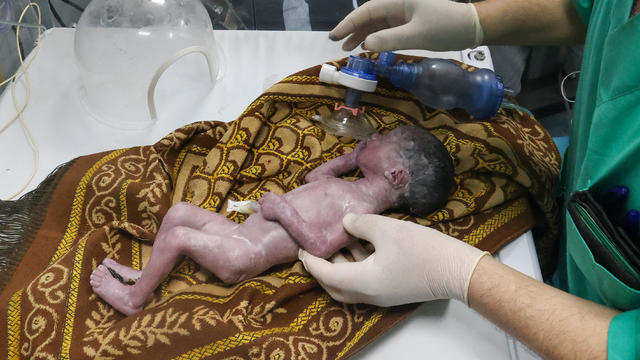
A tiny baby rescued from the womb after an Israeli airstrike in Gaza killed her mother is doing well.

The strike hit a residential building in the western Tel Sultan neighborhood, according to Gaza's civil defense.

How much money can you save by paying off $20,000 in credit card debt with your home equity? Find out now.

A mortgage loan denial is disheartening, but there are ways to improve your chances of future approval.

If you're looking for ways to resolve your overwhelming credit card debt, these strategies are worth considering.

UnitedHealth said it paid the criminals behind attack that crippled hospitals and pharmacies to protect sensitive patient data.

Customers who rely on government assistance programs can get same perks as Prime members, for less.

Cancer, heart disease, respiratory illnesses and kidney dysfunction among the health consequences of a warming planet.

A bill that could ultimately ban TikTok in the U.S. will soon head for a vote in the Senate. Here's what experts say to expect next.

UAW claims historic victory, with an overwhelming majority of VW workers at Chattanooga factory voting to unionize.

Receive a $40 Digital Costco Shop Card when you join as a new member at Costco.com when entering PARA24 at checkout.

Splurge on a luxe Rimowa aluminum suitcase in 2024 and invest in a lifetime of travel.

Discover the best wireless headphones that support spatial audio.

David Pecker, the former publisher of the National Enquirer, detailed an alleged "catch and kill" scheme in which his paper would buy the rights to damaging stories about former President Donald Trump during his 2016 campaign and never publish them. Pecker testified it was this scheme that ultimately led to the payment to adult film star Stormy Daniels. Robert Costa reports.

Dozens of arrests have been made in recent days as police moved to clear pro-Palestine protesters at several colleges throughout the U.S. Nancy Chen has the latest.

President Biden campaigned in Florida just a little over a week before the state's new abortion restrictions are set to take effect. Mr. Biden placed the blame on former President Donald Trump and his three Supreme Court justice picks for the rollback of abortion rights in several states. Nancy Cordes has more.

An American tourist could face at least 12 years in prison in Turks and Caicos after airport security allegedly found ammo in his carry-on bag. Kris Van Cleave has the story.

Jim Axelrod dives into the world of romance scams, showing how sometimes the victims can also become unwitting accomplices in the scammers' financial crimes.

David Pecker, the former publisher of the National Enquirer, detailed an alleged "catch and kill" scheme in which his paper would buy the rights to damaging stories about former President Donald Trump during his 2016 campaign and never publish them. Pecker testified it was this scheme that ultimately led to the payment to adult film star Stormy Daniels. Robert Costa reports.

Dozens of arrests have been made in recent days as police moved to clear pro-Palestine protesters at several colleges throughout the U.S. Nancy Chen has the latest.

President Biden campaigned in Florida just a little over a week before the state's new abortion restrictions are set to take effect. Mr. Biden placed the blame on former President Donald Trump and his three Supreme Court justice picks for the rollback of abortion rights in several states. Nancy Cordes has more.

An American tourist could face at least 12 years in prison in Turks and Caicos after airport security allegedly found ammo in his carry-on bag. Kris Van Cleave has the story.

At least 77 students from the women-only college at Cambridge University were recruited to the code breaking station during World War II.

At his lowest moment, U.S. Army veteran and former teacher Billy Keenan found strength in his faith as he was reminded of his own resilience.

Emmy and Tony Award-winning actress Bebe Neuwirth is back on Broadway, starring as Fraulein Schneider in the new revival of "Cabaret."

Chanel Miller, celebrated for her profound memoir "Know My Name," steps into a new creative realm with her children's book, "Magnolia Wu Unfolds It All." The story, both written and illustrated by Miller, follows two young friends on an adventurous quest through New York City to return misplaced socks from Magnolia's parents' laundromat.

First on "CBS Mornings," we're getting a first listen to a never-before-heard song from Aaron Carter. Carter died in 2022 after struggling with addiction and mental health issues. Now, his team and his sister, Angel Carter Conrad, are releasing his previously unheard music. "The Recovery Album" comes out May 24. Part of the proceeds will go to the nonprofit "The Kids Mental Health Foundation," formerly known as "On Our Sleeves."

CBS News is investigating a growing number of fraud cases known as romance scams. Chief investigative correspondent Jim Axelrod explains how victims can unknowingly become perpetrators in the very scams they fall prey to.

The White House is considering declaring a national climate emergency to unlock federal powers and stifle oil development, according to a Bloomberg report. Meanwhile, the Biden administration is announcing several projects this Earth Week. Columbia University Climate School professor Dr. Melissa Lott joins with analysis.

Teachers are in short supply in the U.S., and researchers say there's declining job satisfaction among those who remain. CBS News reporter Bo Erickson examines what's behind the problems.

The U.S. is close to delivering a $61 billion aid package to the Ukrainian military, and Russia's defense minister said Tuesday that Moscow would ramp up its own weapons production in response. William Taylor, former U.S. ambassador to Ukraine, joins CBS News to discuss.

The Federal Trade Commission officially banned new noncompete agreements Tuesday, giving workers who are considering quitting more options. The U.S. Chamber of Commerce plans to sue in response.

At his lowest moment, U.S. Army veteran and former teacher Billy Keenan found strength in his faith as he was reminded of his own resilience.

A surfing accident left New York teacher Billy Keenan paralyzed, but when he received a call from a police officer, his life changed.

The So Much To Give Inclusive Cafe in Cedars, Pennsylvania employs 63 people — 80% have a disability.

A mom was worried about what her son, who has autism, would do after high school. So she opened the So Much To Give cafe, a restaurant in Cedars, Pennsylvania, that employs people with disabilities – and helps them grow.

A mom worried about her son with autism opens an inclusive cafe that employs people with disabilities. The community around Paradise, California, rallies behind a woman whose beloved pet was stolen. Plus, more heartwarming stories.

CBS Reports goes to Illinois, which has one of the highest rates of institutionalization in the country, to understand the challenges families face keeping their developmentally disabled loved ones at home.

As more states legalize gambling, online sportsbooks have spent billions courting the next generation of bettors. And now, as mobile apps offer 24/7 access to placing wagers, addiction groups say more young people are seeking help than ever before. CBS Reports explores what experts say is a hidden epidemic lurking behind a sports betting bonanza that's leaving a trail of broken lives.

In February 2023, a quiet community in Ohio was blindsided by disaster when a train derailed and authorities decided to unleash a plume of toxic smoke in an attempt to avoid an explosion. Days later, residents and the media thought the story was over, but in fact it was just beginning. What unfolded in East Palestine is a cautionary tale for every town and city in America.

In the aftermath of the Supreme Court striking down affirmative action in college admissions, CBS Reports examines the fog of uncertainty for students and administrators who say the decision threatens to unravel decades of progress.

CBS Reports examines the legacy of the U.S. government's terrorist watchlist, 20 years after its inception. In the years since 9/11, the database has grown exponentially to target an estimated 2 million people, while those who believe they were wrongfully added are struggling to clear their names.

It comes over a year after a shooting at a Nashville school killed three children and three adults.

Jurors in former President Donald Trump's trial in New York heard testimony from a former media executive about his efforts to bury negative stories about Trump before the 2016 presidential election.

Ryan Watson could face at least 12 years in prison in Turks and Caicos after airport security allegedly found four rounds of hunting ammo in his carry-on.

This will be the first General Conference since more than 7,600 mostly conservative congregations left the United Methodist Church between 2019 and 2023.

Tesla reports slide in earnings and revenue, but investors cheered by pledge to accelerate rollout of cheaper vehicles.

Tesla reports slide in earnings and revenue, but investors cheered by pledge to accelerate rollout of cheaper vehicles.

Regulators prohibit new noncompetes, which impede millions of U.S. workers from getting a better job.

Customers who rely on government assistance programs can get same perks as Prime members, for less.

UnitedHealth said it paid the criminals behind attack that crippled hospitals and pharmacies to protect sensitive patient data.

Former President Donald Trump could receive a large windfall from his newly public media company, Trump Media & Technology Group.

It comes over a year after a shooting at a Nashville school killed three children and three adults.

Jurors in former President Donald Trump's trial in New York heard testimony from a former media executive about his efforts to bury negative stories about Trump before the 2016 presidential election.

"He's ultimately responsible," former House Speaker Nancy Pelosi said of Israeli Prime Minister Benjamin Netanyahu.

Former New York Rep. George Santos is no longer running for Congress, he announced Tuesday on X.

Trump's bid for sweeping immunity lands before the Supreme Court, which will hear a case Thursday over whether he can face federal charges related to an alleged effort to overturn the 2020 election.

UnitedHealth said it paid the criminals behind attack that crippled hospitals and pharmacies to protect sensitive patient data.

Warmer weather is prime time for ticks that can carry Lyme disease and other illnesses. Here's how to spot them and get rid of them.

Tires emit huge volumes of particles and chemicals as they roll along the highway, and researchers are only beginning to understand the threat. One byproduct of tire use, 6PPD-q, is in regulators' crosshairs after it was found to be killing fish.

Cancer, heart disease, respiratory illnesses and kidney dysfunction among the health consequences of a warming planet.

To reduce recidivism, some rural counties are hiring community health workers or peer support specialists to connect people leaving custody to mental health, substance use treatment, medical services and jobs.

Ryan Watson could face at least 12 years in prison in Turks and Caicos after airport security allegedly found four rounds of hunting ammo in his carry-on.

This will be the first General Conference since more than 7,600 mostly conservative congregations left the United Methodist Church between 2019 and 2023.

"He's ultimately responsible," former House Speaker Nancy Pelosi said of Israeli Prime Minister Benjamin Netanyahu.

Six men have been arrested on suspicion of involvement in the drug's transport, a Swedish customs official said.

The Netzah Yehuda Battalion of the Israel Defense Forces has faced criticism for its conduct. Will the U.S. take action?

Surprise guests, a broken foot and a history-making headliner.

Eric Church is revered as one of country music's most respected figures, often described as Nashville's renegade. But he admits that even after his success, he sometimes still sees himself as an outsider.

Angel Carter Conrad talks about her brother Aaron Carter, his death and how she hopes his legacy and previously unheard music can help others.

Emmy and Tony Award-winning actress Bebe Neuwirth is back on Broadway, starring as Fraulein Schneider in the new revival of "Cabaret."

Chanel Miller, celebrated for her profound memoir "Know My Name," steps into a new creative realm with her children's book, "Magnolia Wu Unfolds It All." The story, both written and illustrated by Miller, follows two young friends on an adventurous quest through New York City to return misplaced socks from Magnolia's parents' laundromat.

NASA's Voyager 1, the first spacecraft to travel beyond our solar system, has started sending information back to Earth again after scientists managed to fix the probe from 15 billion miles away.

Customers who rely on government assistance programs can get same perks as Prime members, for less.

From labor shortages to environmental impacts, farmers are looking to AI to help revolutionize the agriculture industry. One California startup, Farm-ng, is tapping into the power of AI and robotics to perform a wide range of tasks, including seeding, weeding and harvesting.

Secretary of Commerce Gina Raimondo is at the center of a global competition for semiconductor dominance. It's a battle that also puts her at the center of two of the hottest global national security hotspots. Lesley Stahl of 60 Minutes spoke with Raimondo for the broadcast.

Senators give the green light to a foreign aid package that includes a possible ban on TikTok in the U.S. Here's what experts say could happen next.

The White House is considering declaring a national climate emergency to unlock federal powers and stifle oil development, according to a Bloomberg report. Meanwhile, the Biden administration is announcing several projects this Earth Week. Columbia University Climate School professor Dr. Melissa Lott joins with analysis.

NASA's Voyager 1, the first spacecraft to travel beyond our solar system, has started sending information back to Earth again after scientists managed to fix the probe from 15 billion miles away.

Relatively few Americans say they know a lot about President Biden's initiatives to combat climate change, according to a CBS News poll. Carolyn Kissane, a New York University global affairs associate dean and professor, joins CBS News with more on Biden's climate policies.

A photo taken two days after the sinking of the RMS Titanic apparently shows the iceberg that doomed the so-called unsinkable ship in 1912. CBS News' John Dickerson has details.

Despite how terrifying sharks might seem, the creatures are critical to the survival of the world's oceans. Oceans generate 50% of the oxygen on the planet and absorb 90% of excess heat created by global warming. CBS News senior national and environmental correspondent Ben Tracy spoke with conservationists in the Bahamas.

CBS News is investigating a growing number of fraud cases known as romance scams. Chief investigative correspondent Jim Axelrod explains how victims can unknowingly become perpetrators in the very scams they fall prey to.

Jim Axelrod dives into the world of romance scams, showing how sometimes the victims can also become unwitting accomplices in the scammers' financial crimes.

Don Steven McDougal, a family friend, was indicted by a Polk County grand jury in connection with the death of an 11-year-old girl.

Six men have been arrested on suspicion of involvement in the drug's transport, a Swedish customs official said.

Larry Webb confessed to shooting and killing Susan and Natasha "Alex" Carter, who had been missing for 24 years, officials said.

In November 2023, NASA's Voyager 1 spacecraft stopped sending "readable science and engineering data."

In two weeks, Boeing's Starliner spacecraft is scheduled to launch its first piloted test flight, bringing two veteran NASA astronauts to the International Space Station. Astronaut Matt Dominick joined CBS News from the ISS to talk about the mission and life in space.

A process called cryopreservation allows cells to remain frozen but alive for hundreds of years. For some animal cells, the moon is the closest place that's cold enough.

The Lyrid meteor show is set to peak as the week begins.

April's full moon, known as the Pink Moon, will reach peak illumination on Tuesday, but it will appear full from Monday morning through Thursday morning.

A look back at the esteemed personalities who've left us this year, who'd touched us with their innovation, creativity and humanity.

The Francis Scott Key Bridge in Baltimore collapsed early Tuesday, March 26 after a column was struck by a container ship that reportedly lost power, sending vehicles and people into the Patapsco River.

When Tiffiney Crawford was found dead inside her van, authorities believed she might have taken her own life. But could she shoot herself twice in the head with her non-dominant hand?

We look back at the life and career of the longtime host of "Sunday Morning," and "one of the most enduring and most endearing" people in broadcasting.

Cayley Mandadi's mother and stepfather go to extreme lengths to prove her death was no accident.

CBS News is investigating a growing number of fraud cases known as romance scams. Chief investigative correspondent Jim Axelrod explains how victims can unknowingly become perpetrators in the very scams they fall prey to.

The White House is considering declaring a national climate emergency to unlock federal powers and stifle oil development, according to a Bloomberg report. Meanwhile, the Biden administration is announcing several projects this Earth Week. Columbia University Climate School professor Dr. Melissa Lott joins with analysis.

Teachers are in short supply in the U.S., and researchers say there's declining job satisfaction among those who remain. CBS News reporter Bo Erickson examines what's behind the problems.

The U.S. is close to delivering a $61 billion aid package to the Ukrainian military, and Russia's defense minister said Tuesday that Moscow would ramp up its own weapons production in response. William Taylor, former U.S. ambassador to Ukraine, joins CBS News to discuss.

Jim Axelrod dives into the world of romance scams, showing how sometimes the victims can also become unwitting accomplices in the scammers' financial crimes.